What Is a Double Displacement Reaction?
Let’s imagine ourselves going to a dance and having a dance partner. Once we arrive, we interact with the other people, and we end up switching dance partners with another person. Now you end up with a new dance partner. The same kind of thing can be compared to what occurs in a double displacement reaction in chemistry.
A double displacement reaction, also known as a double replacement reaction or metathesis, is a type of chemical reaction where two compounds react, and the positive ions (cation) and the negative ions (anion) of the two reactants switch places, forming two new compounds or products. Here, you can see the general form of a double displacement reaction:
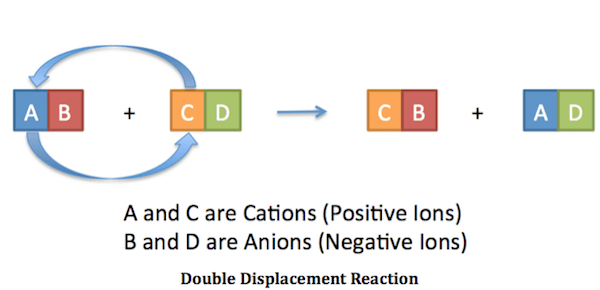 |
How to Complete a Double Displacement Reaction
Just like how dance partners can be switched, the products of a double displacement reaction are the result of the cations and anions of the reactants trading partners with each other. Now we’ll learn the steps to complete and predict the correct products for a double displacement reaction. Let’s start with a look at the chemical reaction between Na 2 S and HCl.
 |
Step 1: Identify the Individual Ions from the Reactants and Their Charges
For the reactant Na 2 S , there is a cation (positive ion) and an anion (negative ion). Na is written first, so that means Na is the cation. S is written second, so S is the anion. Cations and anions have charges that are either positive or negative integers. A cation has a positive charge, and an anion has a negative charge.
S has no subscript, which is just the small numbers at the bottom right after each element. No subscripts mean that the subscripts are equal to one. This means that if we rewrite the compound Na 2, the subscript for S is 1. We can then reverse the subscripts and figure out the individual charges of Na and S. Na has a charge of +1, and S has a charge of -2, as shown here:
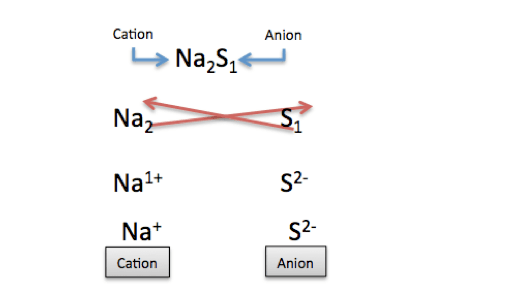 |
Now, let us take a look at the other reactant, HCl. The cation is H, and the anion is Cl. Like we did with Na 2 S , we reverse the subscripts to figure out the individual charges.
 |
Step 2: Switch the Cations and Anions of the reactants
To predict the products, bring down the charges as shown in this illustration:
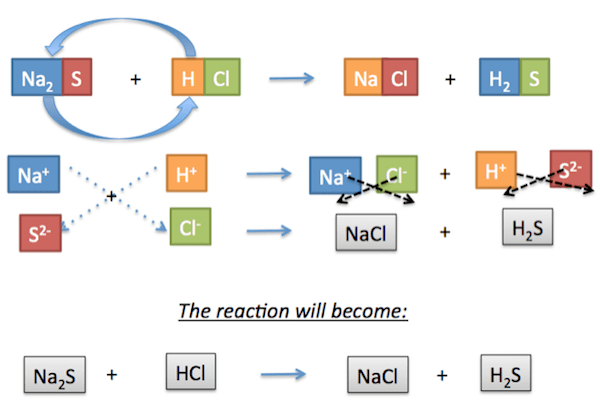 |
Originally, the pairs are Na-S and H-Cl. Now, the new partner of Na is Cl, and the new partner of H is S.
Step 3: Balance the Chemical Reaction
The reaction in this case is not balanced, because the number of Na and H atoms (in red) is not the same.
 |
What we need to do next is to balance the reaction by adding coefficients, which are the numbers before each compound. In this case, we will put a 2 in front of HCl to balance the H atoms and a 2 in front of NaCl to balance the Cl atoms.
 |
Now, we have successfully balanced the reaction.
Examples of Double Displacement Reactions
For our first example, we’ll look at the reaction between Li 2 SO 4 and BaCl2 . SO4 is a polyatomic ion, so we will treat this as one anion. The ion SO4 does not have a subscript (4 is not the subscript because this is included in the ion). The subscript of SO4 is 1. Let’s first determine the ions and their charges.
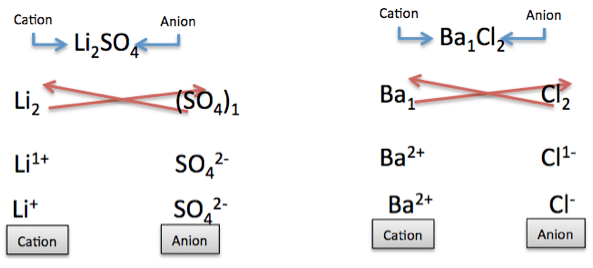 |
Now, we can switch the ions and come up with the double displacement reaction.
 |
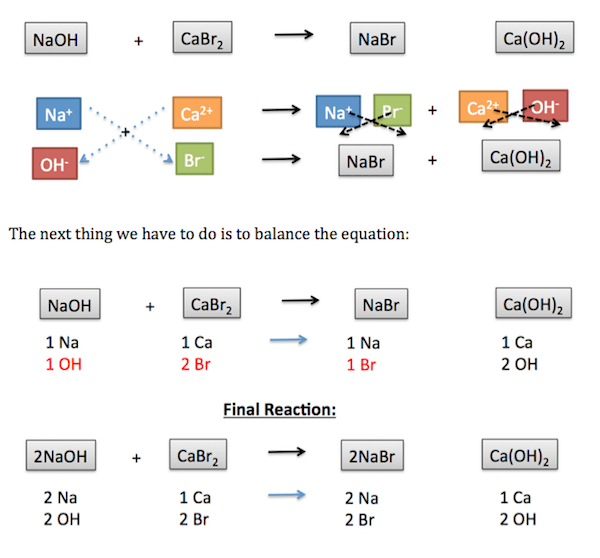
For our next example, let’s look at the reaction between NaOH and CaBr2 . For this reaction, OH is a polyatomic ion and is treated as one whole anion. The individual ions and the charges for these two reactants are demonstrated in this image:
 |
Now, we can switch the ions, as you can see here:
 |
Types of Double Displacement Reactions
Now that we have gone over the steps to complete and balance double displacement reactions, let us go over the different types of double displacement reactions. There are three types of double displacement reactions: precipitation, neutralization and gas formation. We will discuss each and go over an example for each.
Precipitation Reaction
A precipitation reaction is when two compounds react and form a precipitate, which is a solid product. This product is insoluble, or cannot be dissolved in water.
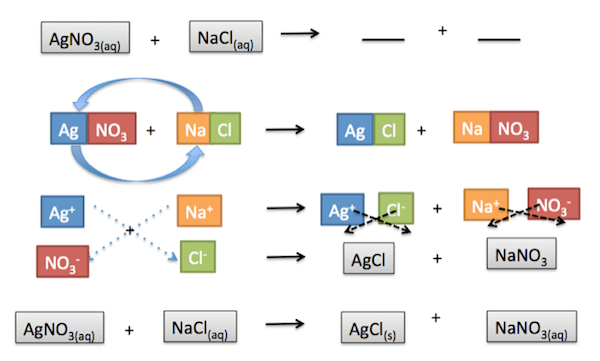
For example, we have the reaction between NaCl and AgNO3 . When the two solutions, NaCl and AgNO3 , are mixed, a precipitate, AgCl, is formed. The subscript aq means aqueous, which means it is a solution in a solvent. The subscript s means solid. These subscripts indicate the phase of the compounds.
Going over the steps we outlined earlier to complete this double displacement reaction, we obtain the final reaction at the bottom of this image. The precipitate formed is AgCl, as indicated by the subscript s. The resulting reaction is already balanced, so there is no need to add coefficients.
 |